The Association for the Advancement of Medical Instrumentation (AAMI) generates numerous standards and guidelines used by professionals in the healthcare industry. Occasionally, the AAMI Standards board will provide additional guidance to specific standards in the form of a Technical Information Report (TIR). These TIRs reflect common industry practices that evolve from an accumulated process knowledge base.
In the last few years many changes have occurred to the guidance documents we have all followed to set minimum sterilization doses. With the completion of ANSI/AAMI/ISO 11137:2006 and the addition of AAMI TIR 33, many options were placed in the hands of the healthcare industry. Significant changes, such as bringing VDmax 25 into an ISO document, the additional of a VDmax 15 option, and incorporation of single lot validation for Method 1, have eliminated or reduced the need to use older AAMI or AAMI/ISO documents such as TIR 27, 15844 and 13409 . These changes have also made the familiarity with the current ANSI/AAMI/ISO documents even more critical to the needs of the healthcare industry.
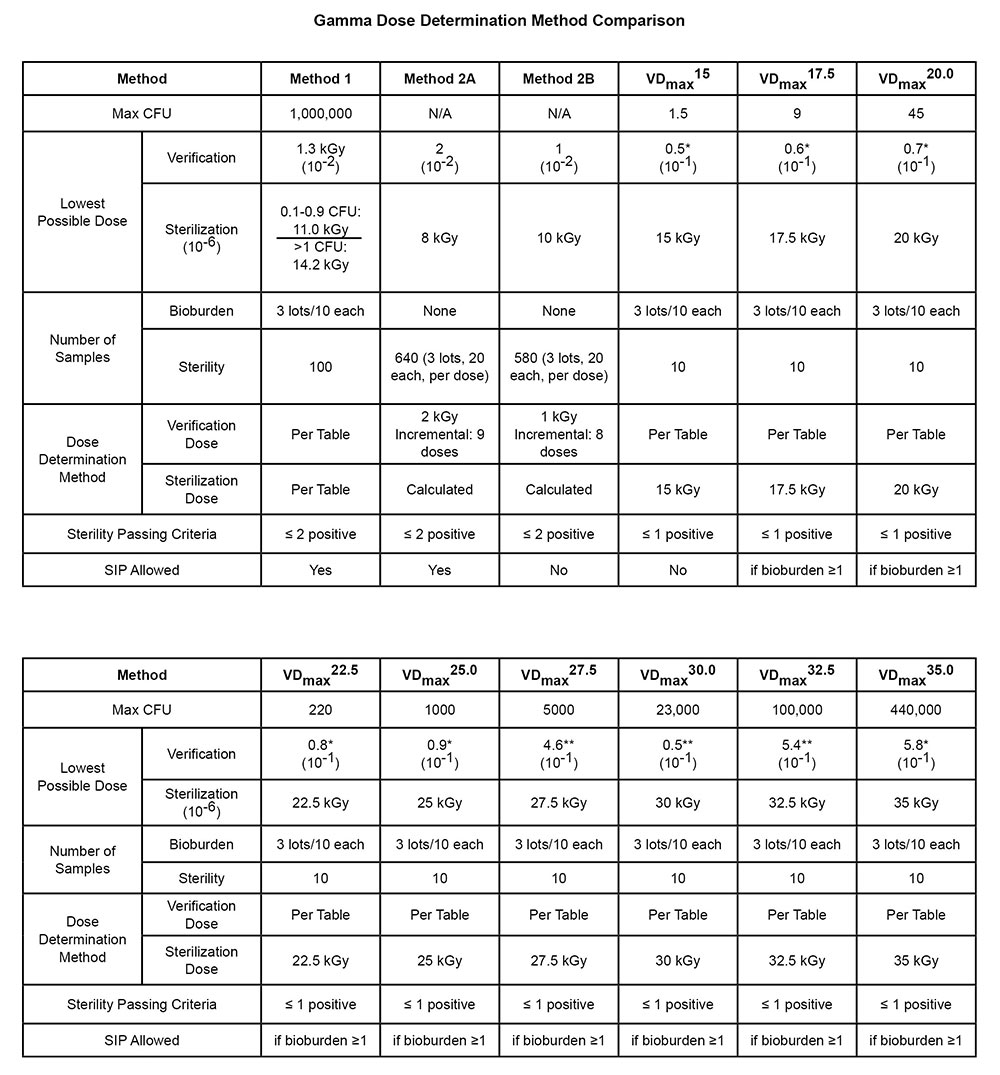
While this document is not intended to be an exhaustive comparison of old versus new guidelines, we wanted to bring together in a chart format a comparison of the methods available as starting point when preparing to review these documents for the first time as they apply to your specific product needs or requirements. This TechTip will look at dose setting, while a companion document will look at dose audits in the same format.
References:
- ANSI/AAMI/ISO 11137-1:2006. Sterilization of health care products – Radiation-Part 1: Requirements for development, validation, and routine control of a sterilization process for medical devices.
- ANSI/AAMI/ISO 11137-2 :2006. Sterilization of health care products – Radiation-Part 2: Establishing the sterilization dose.
- ANSI/AAMI/ISO 11137-3:2006. Sterilization of health care products – Radiation-Part 3: Guidance on dosimetric aspects.
- AAMI TIR 33:2005. Sterilization of healthcare products-Radiation-Substantiation of a selected sterilization dose-Method VDmax.