Background
A verification (dose audit) or sterilization dose experiment requires a narrow and specific dose range to be applied to the samples used for tests of sterility as evidence that the routine minimum dose remains appropriate for delivering the require sterility assurance level.
Dose requirements for radiation processing are defined in ISO 11137 part 2 ((7.2.5 (Method 1) and 9.2.5 (VDmax method)). Due to the penetration capability of the electrons, this narrowdose range is sometimes not process capable for some types of devices/products.
Therefore, using electron beam to perform the verification dose experiment increases the risk of a nonviable process, not due to the bioburden level, but as a result of the entire sample not
receiving the desired dose range.
Nevertheless, the routine electron beam process dose specification of those devices may demonstrate a desired penetration that is evidenced using dosimetry as part of the Performance Qualification (PQ) in accordance with ISO 11137.
As the purpose of the dose verification experiment is to demonstrate that the verification dose is capable of delivering a pre-defined inactivation (10-1 or 10-2, or greater) of the product’s natural microbiological challenge, the verification may be performed using a radiation source capable of delivering the required dose as defined in ISO 11137-2.
Therefore, for those products where the specified dose cannot be achieved, the dose verification experiment may be performed with photon technology (gamma or X-ray) where penetration capability is higher and will allow for a better homogeneity of delivered dose throughout the material being irradiated.
Transference of the Verification Dose or Sterilization Dose
1. According to ISO 11137-1 (section 8.4.2), transference of the verification dose or sterilization dose to another radiation source is possible when data are available to demonstrate that differences in operating conditions of the two radiation sources have no effect on microbial effectiveness.
2. There is published evidence that inactivation of microorganisms is a result of only the absorbed dose and not of the technology delivering the dose.
The following referenced publications arrive to the same conclusion that the sterilization dose is not dependent of the radiation sources:
• Radiation Sterilization: Dose Is Dose. Joyce M. Hansen, Niki Fidopiastis, Trabue Bryans, Michelle Luebke and Terri Rymer AAMI : Industrial Sterilization : Process Optimization and Modality Changes – 2020
• Microbicidal effectiveness of X-rays used for sterilization purposes Tallentire, A. and Miller, A. (2015) Radiation Physics and Chemistry, 107, pp. 128–130 doi: 10.1016/j.radphyschem.2014.09.012
• A comparison of the microbicidal effectiveness of gamma rays and high and low energy electrons radiations Alan Tallentire, Arne Miller, Jacob Helt-Hansen Radiation Physics and Chemistry 79 (2010) 701-704
In addition, AAMI TIR104 Guidance on transferring health care products between radiation sources, section 5.3, states the following:
Tallentire, Miller and Helt-Hansen verified that a representative D10 value for a microorganism known to be radiation resistant did not vary among gamma and electron beam irradiation and agreed with previously published values. Tallentire and Miller expanded that study to include X-rays, showing that the D10 value was consistent across all three radiation modalities. A paper published by Hansen, Fidopiastis, Bryans, Luebke and Rymer evaluated microbial radiation resistance across a matrix of differing radiation modalities, microbial challenges, and dose rates, with the result that no significant differences were seen in the rate of microbial lethality across the range of radiation modalities and dose rates evaluated. These studies and practices indicate that the operating conditions of different irradiators, specifically as related to dose rate and
radiation modality, do not have a discernable effect on microbial effectiveness, such that the transfer of sterilization dose can be made to the candidate irradiator without further assessment and comply with the limitations specified in ANSI/AAMI ISO 11137-1.
However, two situations can result in the need for further assessment to determine if transferring sterilization or verification doses to the candidate irradiator is warranted:
1. The presence of liquid/water in the health care product; and
2. Composition of the health care product that supports microbial growth.
TIR104 includes further guidance as a flow chart below:
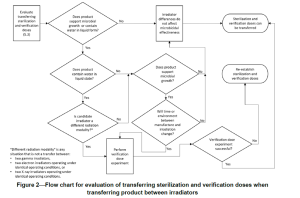
Proposed method when a transfer is required:
1. If a verification dose request for use of electron beam can be performed by electron beam within specification, continue with electron beam.
2. If the PQ shows the process is not capable using electron beam, assess an alternative radiation source.
3. If the alternative radiation source can deliver the dose within specification, perform a verification dose request at the alternative radiation source.
Note: Do consider any changes to the processing of samples, e.g. transportation and storage conditions.
As explained in ISO 11137-1, A 8.4.2.2, the comparison to demonstrate that transference does not alter microbial effectiveness can be accomplished by the performance of a successful verification dose experiment using the radiation source to which transfer is being considered. If the demonstration is successful, the verification and sterilization dose can therefore be transferred to the other technology.
Appendix:
9.2.5 Stage 4: Perform verification dose experiment
9.2.5.1 Select 10 product items from a single batch of product. The 10 product items for the performance of Stage 4 may be selected from one of the batches on which a bioburden determination was carried out in Stage 2 or from a fourth batch manufactured under conditions that are representative of normal production (see 5.3).
9.2.5.2 Irradiate these product items at VDmax25 by more than 10%.
The arithmetic mean of the highest and lowest doses to product items should not be less than 90% of VDmax25.
Determine the dose delivered (see 5.5).
If the highest dose to product items exceeds VDmax25 by more than 10%, the verification dose experiment shall be repeated.
If the arithmetic mean of the highest and lowest doses to product items is less than 90% of VDmax25, the verification dose experiment may be repeated. If this mean dose is less than 90% of VDmax25 and, on performance of tests of sterility, acceptable results are observed (see 9.2.6.1), the verification dose experiment need not be repeated.
*Note. Extract of “ISO 11137-2: Sterilization of health care products – Radiation – Part 2: Establishing the sterilization dose.”
“ISO 11137-1: Sterilization of health care products – Radiation – Part 2: requirements for the development, validation and routine control of a radiation sterilization process for medical devices.” Annex 8.4.2 gives additional information:
A.8.4.2 Transference of verification or sterilization dose
A..4.2.1 There is a concern in transferring between types of radiation sources with widely differing dose rates that can provide different microbicidal effects. Demonstrating that the microbicidal effectiveness is not affected by the change in dose rate provides the necessary data for the transference to be permitted.
EN ISO 11137-1 (E)
A.8.4.2.2 Experimental evidence indicates that when irradiation occurs under “dry” conditions, microbicidal effectiveness is independent of the operating conditions of the sources; hence the granting of this permission.
With regard to item 8.4.2.2 b), a difference in dose rate is of concern when transferring between types of radiation sources; dose rate can alter the microbicidal effectiveness. This comparison to demonstrate that transference does not alter microbial effectiveness can be accomplished by the performance of a successful verification dose experiment (see ISO 11137-2) using the radiation source to which transfer is being considered.
References:
1. ISO 11171-1:2020 “Hydraulic fluid power — Calibration of automatic particle counters for liquids Part 1 and 2”
2. AAMI TIR104:2022 “Guidance on transferring health care products between radiation sterilization sources”
3. Tallentire, A, Miller, A, Helt-Hansen, J. (2010). A comparison of the microbicidal effectiveness of gamma rays and high and low energy electron radiation., Radiation Physics and Chemistry, 79 :701-704
4. Hansen, JM, Fidopiastis, N, Bryans, T, Luebke, M, and Rymer, T. (2020). Radiation Sterilization : Dose Is Dose. Biomedical Instrumentation & Technology : Industrial Sterilization : Process Optimization and Modality Changes, Vol. 54 (s1), 45-52
5. Tallentire, A. and Miller, A. (2015). Microbicidal effectiveness of X-rays used for sterilization purposes. Radiation Physics and Chemistry, 107, pp. 128–130.
Related TechTips
Transfer of Ionizing Radiation Technologies for Medical Devices Based on Guidance in ISO 11137-1, ISO 13004, and AAMI TIR 104
This TechTip will address transfer types and areas of consideration. Considerations should also be given to specific product requirements and registrations, as the information provided is based primarily on guidance in ISO 11137 and may not cover all the requirements and registrations specific to a manufactured product.
Considerations When Establishing a Maximum Sterilization Dose for Radiation Processing
Per ISO 11137, products sterilized by X-ray, gamma, and E-beam irradiation require a documented and validated dose range. A dose range is composed of: A minimum dose based on the microbiologic considerations (type and quantity of organisms and their re
Irradiations Effect on Cannabis
Background As an agricultural product, cannabis can be exposed to contamination with pathogenic fungi, bacteria, yeast, and mold during growing, drying, packaging, and/or delivery, which may put consumers at health risks. For example, fatal pulmonary A
Radiation Sterilization Master File Pilot Program FAQ
Q1: What is the Radiation Sterilization Master File Pilot Program? A:The Radiation Sterilization Master File Pilot Program (Pilot Program) is a voluntary program that intends to allow companies that terminally sterilize single-use medical devices using
