Sustainable EO® Sterilization
Sustainable EO® Sterilization
Ethylene oxide (EO) is a widely accepted sterilization technology that has delivered safe, sterile healthcare products for patient care since the 1960s. This low temperature, gaseous process can efficiently penetrate surfaces of most medical devices and is ideal for a wide variety of materials.
What is the Sustainable EO Sterilization Services Program?
As part of our commitment to providing innovative and sustainable services, STERIS AST has developed the Sustainable EO sterilization services program. Our Sustainable EO services provide Customers with strategies to reduce the EO sterilant used in the sterilization process to achieve the prescribed Sterility Assurance Level.
The program includes innovative approaches to EO sterilization, such as cycle design, validation strategy, and process challenge device design, all focused on the reduction of EO residuals on healthcare products.
What are the Main Benefits of Optimized Sterilant Input?
Optimized sterilant input may provide several benefits, including:
- Lower product residuals to meet current and future patient safety requirements
- Improved occupational safety
- Improved supply chain efficiencies due to reduced aeration (off-gassing) of ethylene oxide gas
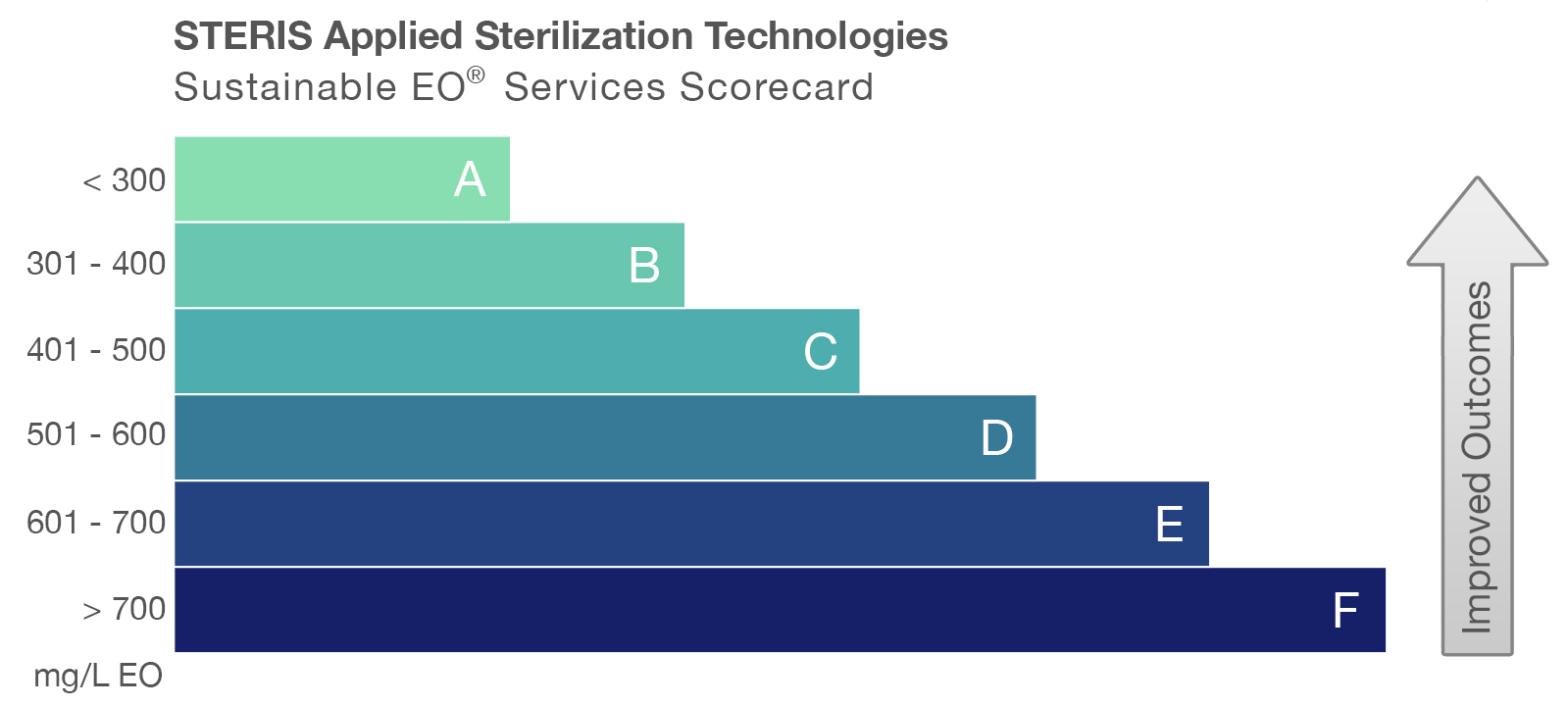
Our EO TechTeam® professionals support manufacturers of healthcare products with validating the Sustainable EO sterilization cycles for their unique products. Upon completion of the validation, a final report is provided that includes the Sustainable EO Services Scorecard.
The scorecard, which can also be provided as a benchmark for existing sterilization cycles, highlights the achieved sustainability rating by showing the EO concentration (mg/l) used in each cycle.
Go here to learn how the Ethylene Oxide Master File Pilot Program may help Customers modify existing EO process through the STERIS Sustainable EO sterilization program, without the need to submit a PMA supplement to the FDA for approval.
Interested in Learning how Sustainable EO Sterilization Services can be Applied to your Product?
Contact STERIS AST using the form on this page or by calling:
Americas: 877-783-7479
EMEAA: +44 330 236 8344